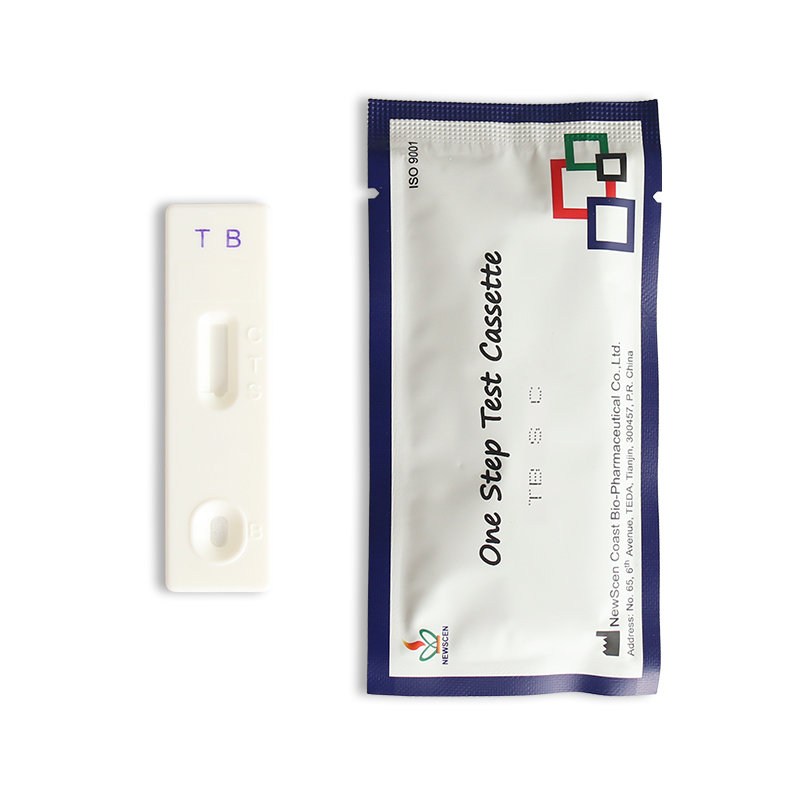
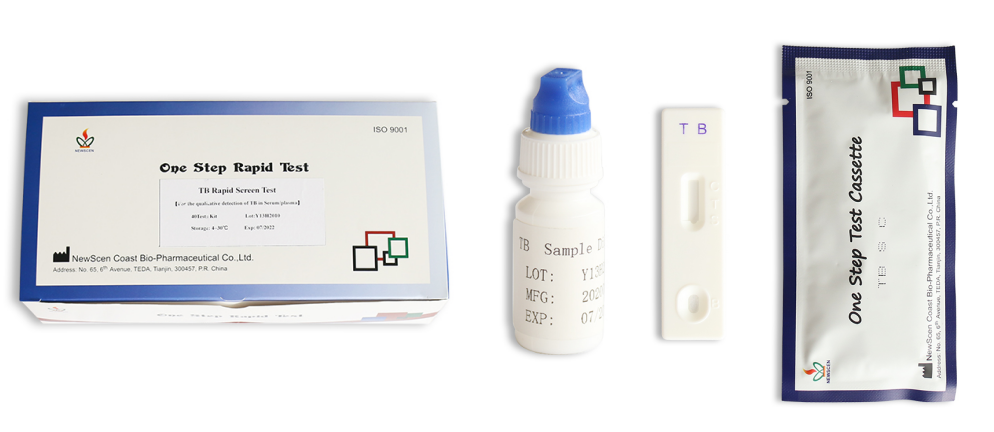
Tuberculosis Antibody (TB) Test Kit
This product is for in vitro qualitative detection of M. tuberculosis (M. tb) antibodies in human serum samples. This product is suitable for the auxiliary diagnosis of tuberculosis. Tuberculosis is caused by Mycobacterium tuberculosis and is a systemic infectious disease, of which tuberculosis is the most, accounting for about 90%. The diagnosis of tuberculosis is mainly based on bacteriology laboratory examinations, combined with chest imaging, epidemiology, and clinical manifestations, necessary auxiliary tests, and differential diagnosis, and made by comprehensive analysis.
- Product Details
- Technical Specification
Sample type: Serum, Plasma
Detection type: Qualitative
Method: Colloidal Gold Rapid Test
Usage/Application: Laboratory / Hospital / Pathology
Function: Diagnose
Certificate: ISO9001/ ISO13485/ CE by TUV
Format: Strip, Cassette, Midstream
Reading time: 15 minutes
Packaging Details:
Pouch+Box+Carton packaging
(1) With our company’s Logo
(2) With the natural package
(3) With OEM package
(4) ODM
①For Professional Use: 40 Cassette/Kit; 100 Strips/Kit; 50 Strips/Bottle (For Customization)…
②Uncut Sheet for OEM
Diagnostic Kit for M. Tuberculosis Antibody (TB) (Colloidal Gold) is intended for qualitative in-vitro detection of antibodies to Mycobacterium tuberculosis in human serum.
It applies to auxiliary diagnosis of tuberculosis.
* High Sensitivity
*Specificity
*Reliable: high accurate, early detection of the presence of TB
*Simple: No Instrument Required
*Convenient: Room Temperature Storage, Built-In Control line
*Fast: Results in 5-10 minutes, strong positive results may be observed promptly within 3 minutes
* Certified by Authoritative Certification System and Standards
*Winner of “the National Rapid Diagnostic Kit for Clinical Performance Assessment”
Storage:
The kit should be stored at room temperature (4-30°C, do not freeze) for 18 months from the date of manufacture. Keep the test cassette in a sealed pouch until use. Once you have taken the test cassette out of the pouch, perform the test as early as possible (within 1 hour) to avoid the test cassette from becoming moist. Do not use the test beyond the indicated expiration date.
The diluent buffer should be stored at room temperature (4-30°C, do not freeze).
Procedure of NewScen Tuberculosis Antibody (TB) Test Kit:
1. Place the test cassette on a flat surface. Use it immediately once unsealed.
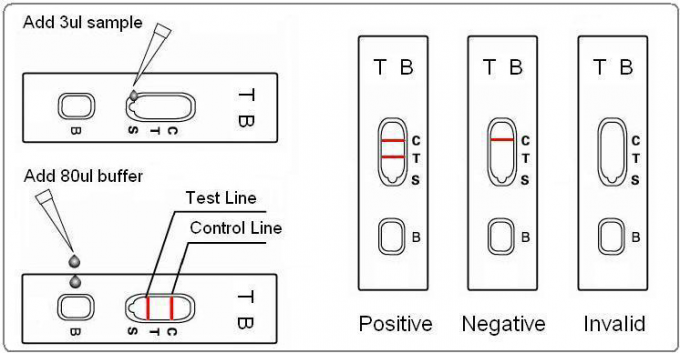
2. Open the pouch and add a 4μL specimen (serum/plasma) into the middle position of the NC membrane area of the cassette.
3. After the sample is completely absorbed, add 2 drops (50~80μL) of diluent buffer to the sample hole and start the timing.
4. Observe the result in 15 minutes.
Result Interpretation:
1. Negative: No red line appear in the test zone (T) in 15 minutes only a red line shows in the control zone (C), indicates that no TB antibodies have been detected with this test.
2. Positive: One red line shows in the control zone (C) and one in the test zone (T) indicates the specimen infects TB possibly, recommend further testing.
3. Invalid: No red lines appear in the control zone (C) indicates that the test is invalid. Discard the test cassette and perform with a new cassette.
Built-In Control
The kit has a built-in procedural control that demonstrates assay validity. If a red line appeared in the control zone (C), it indicates that the test runs correctly.
Limitation:
1. The kit is intended for the qualitative detection of antibodies to TB.
2. The positive result cannot be the final diagnosis of TB. Any positive result must be interpreted in conjunction with the patient clinical history and other laboratory testing results. Follow-up and supplementary testing of all positive specimens with other tests are required to confirm any reactive result.
3. The kit is a qualitative assay and the results cannot be used to measure the concentration of antibodies.

 38m Waxfilm
38m Waxfilm  200ul sample tip
200ul sample tip  1000ul Wide Orifice Pipette Ti...
1000ul Wide Orifice Pipette Ti...  10ul microinjection capillary ...
10ul microinjection capillary ...  10ul extended tip
10ul extended tip  Viral DNA/RNA Kit
Viral DNA/RNA Kit  Vacuette Blood Collection Tube...
Vacuette Blood Collection Tube...  Pathogenic Microorganism DNA/R...
Pathogenic Microorganism DNA/R...  NGS TPH DNA Library Prep Set f...
NGS TPH DNA Library Prep Set f...  Magbead DNA Purification Kit (...
Magbead DNA Purification Kit (...  Monkeypox IgG/IgM Rapid Test C...
Monkeypox IgG/IgM Rapid Test C...  Hcg Pregnancy Test Uncut Sheet
Hcg Pregnancy Test Uncut Sheet 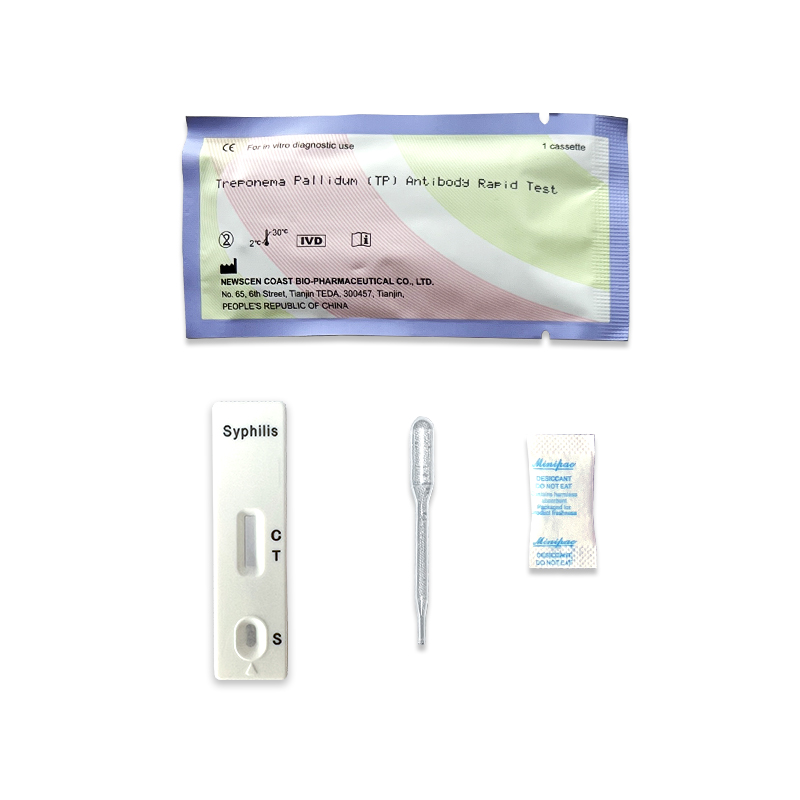 TP (Treponema Pallidum/Syphili...
TP (Treponema Pallidum/Syphili... 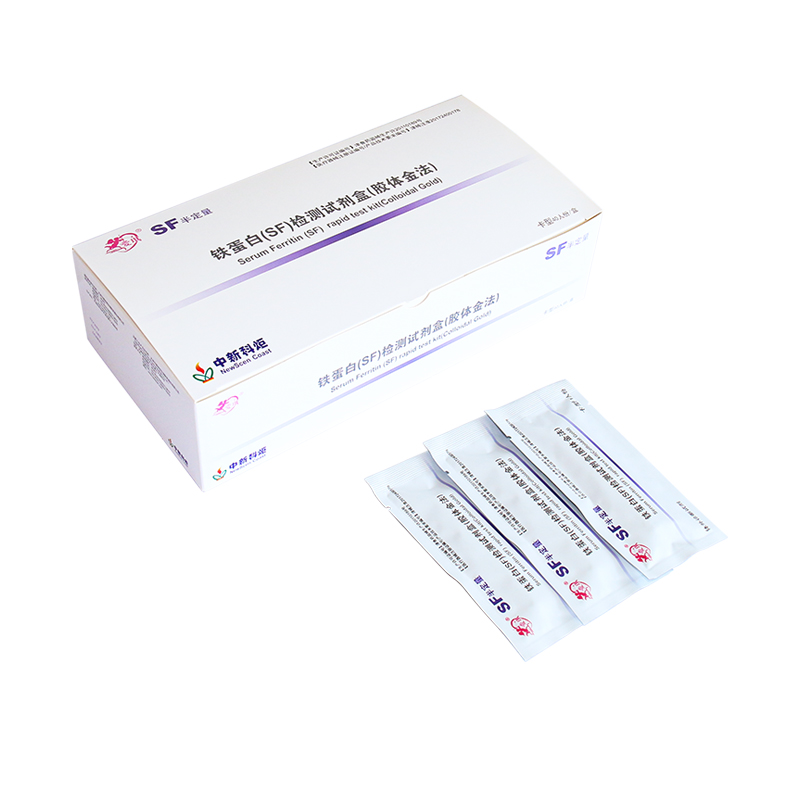 Serum Ferritin (SF) quantitati...
Serum Ferritin (SF) quantitati... 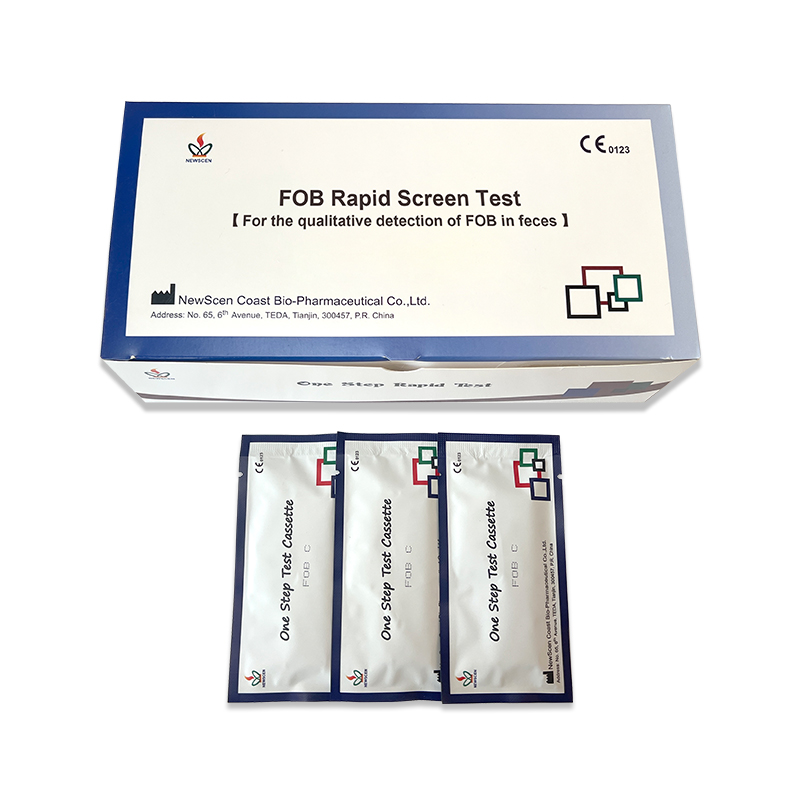 Fecal Occult Blood Fob Rapid T...
Fecal Occult Blood Fob Rapid T... 





Pablo Villalpando –
Good product. I really like it so much.