(CRP) Whole course C-reactive protein detection card
The kit is used for quantitative determination of C-reactive protein in human serum, plasma or whole blood in vitro.
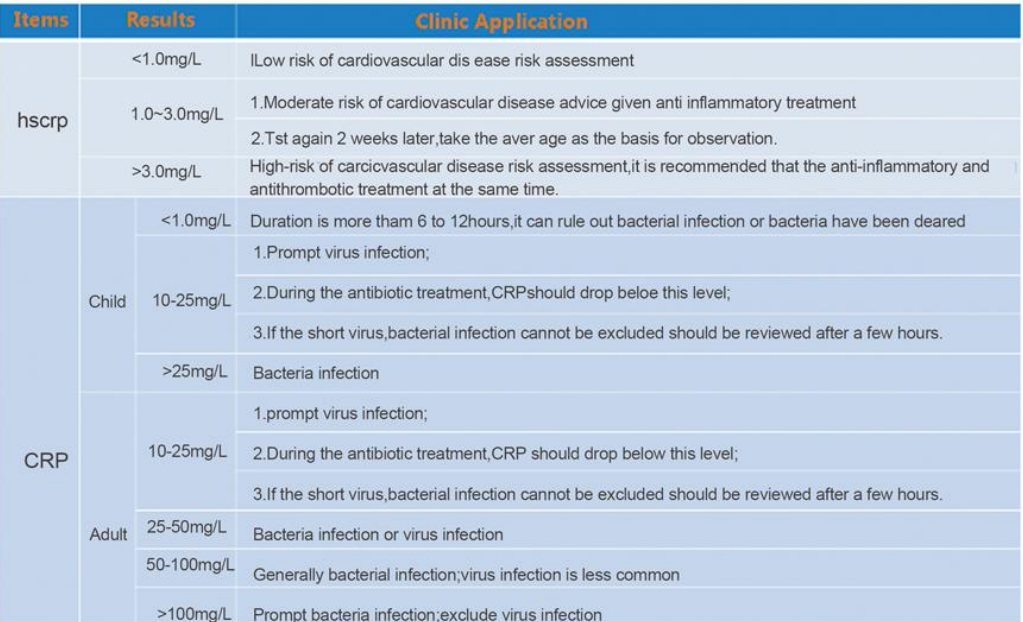
It is of great significance to determine CRP content in human blood to distinguish bacterial and viral infection.
- Product Details
- Technical Specification
Packing Specification: 10 / box, 20 / box, 50 / box
Sample Requirement
No special treatments are needed, conventional method is used to collect whole blood sample, or centrifugally separate serum/ plasma sample. When used in 24 hours, the sample can be preserved in 2~8 °C, for long-time storage, the samples shall be kept under - 20 °C (no more than 6 months) and avoid repeated freezing. Please do not use severe hemolysis, blood lipid, or jaundice specimen.
Reference Value
Each laboratory should establish its own normal value, the following value is only for reference. Through verification of 180 cases clinical samples, the kit establish its own normal reference value. Reference normal value: <10pg/ml.
Interpretation of the Testing Result
1. Each laboratory should establish its own normal and clinical pathological value range, the normal range above mentioned just for your reference.
2. The test result of this kit is used only for clinical auxiliary diagnosis, not the only supporting evidence for clinical diagnosis. Other clinical symptoms and detection index shall be used for comprehensive diagnosis.
The Limitations of Testing
1. Autoimmune antibodies and heterophile antibody in the serum may interfere with the testing results.
2. This kit accurate measurement range should not exceed the concentration range of standard curve. If the sample exceeds the curve, it needs to be properly diluted to get accurate test results.
3. The test results by other assay methods have no direct comparability.
Product Performance
1. Physical Performance: the test strip should be neat and complete, no burr, no damage, no pollution; materials attached firmly; the width of the test strip >3mm; fluid migration velocity should be not less than 10 mm/min.
2. Accuracy: test the sample provided by WHO, the relative deviation should be within ±15%.
3. Linear: linear range is [0.5, 200] pg/ml, correlation coefficient (r) shall be not less than 0.99.
4. Repeatability: Repeatability (CV %) should be no higher than 15.0%.
5. Reproducibility: Reproducibility (R %) should be no higher than 15.0%.
6. Blank detection limit: LOQ should be no higher than 0.5卩g/ml.
7. Detect cross reaction substance of 3pg/ml PCT, the result should be<0.5pg/ml.
Cautions
1. Test must comply with the laboratory management norms, and strict to prevent cross contamination. All samples and all kinds of waste should be disposed as the infection.
2. Read the instructions carefully before operation, in strict accordance with the manual operating procedures, different batches of reagent and test strip cannot mix, keep the kit in dry and clean place.
3. Pipetting sample should be slow and accurate.
4. After pipetting the sample, put the test strip on the laboratory bench, waiting for reaction.
5. The reaction time is 10 minutes, error is less than 1 minute.

 38m Waxfilm
38m Waxfilm  200ul sample tip
200ul sample tip  1000ul Wide Orifice Pipette Ti...
1000ul Wide Orifice Pipette Ti...  10ul microinjection capillary ...
10ul microinjection capillary ...  10ul extended tip
10ul extended tip  Viral DNA/RNA Kit
Viral DNA/RNA Kit  Vacuette Blood Collection Tube...
Vacuette Blood Collection Tube...  Pathogenic Microorganism DNA/R...
Pathogenic Microorganism DNA/R...  NGS TPH DNA Library Prep Set f...
NGS TPH DNA Library Prep Set f...  Magbead DNA Purification Kit (...
Magbead DNA Purification Kit (...  Monkeypox IgG/IgM Rapid Test C...
Monkeypox IgG/IgM Rapid Test C...  Hcg Pregnancy Test Uncut Sheet
Hcg Pregnancy Test Uncut Sheet  TP (Treponema Pallidum/Syphili...
TP (Treponema Pallidum/Syphili...  Serum Ferritin (SF) quantitati...
Serum Ferritin (SF) quantitati...  Fecal Occult Blood Fob Rapid T...
Fecal Occult Blood Fob Rapid T... 

Pablo Villalpando –
Good product. I really like it so much.